| Test | Certification | Status |
| Biocompatibility | 21 CFR Part 58 Compliance | |
| Good Laboratory Practice for Nonclinical / Lab Studies | ||
| ISO 10993-13 tests | Passed | |
| Performance testing | Biomechanical- static and cyclic loading | |
| USP 39-NF 33:2016 <881> Tensile Strength | Passed | |
| Degradation Studies | ASTM F1635 | Passed |
| Sterilization Validation | ISO 11135 | Passed |
| EO Residuals | ISO 11135 | Passed |
| Shelf life -post accelerated aging | ASTM F1980 | Passed* |
| Real Time Shelf Life testing | ISO 11607 -1:2006 | Passed* |
| Seal Package Validation | ASTM F88/F88M-15 | Passed |
| Bubble burst testing | ASTM 2096 – 11 | Passed |
| Extraction and Leaching | ISO 10993-12 and 10993-18 | Passed |
*1 year passed


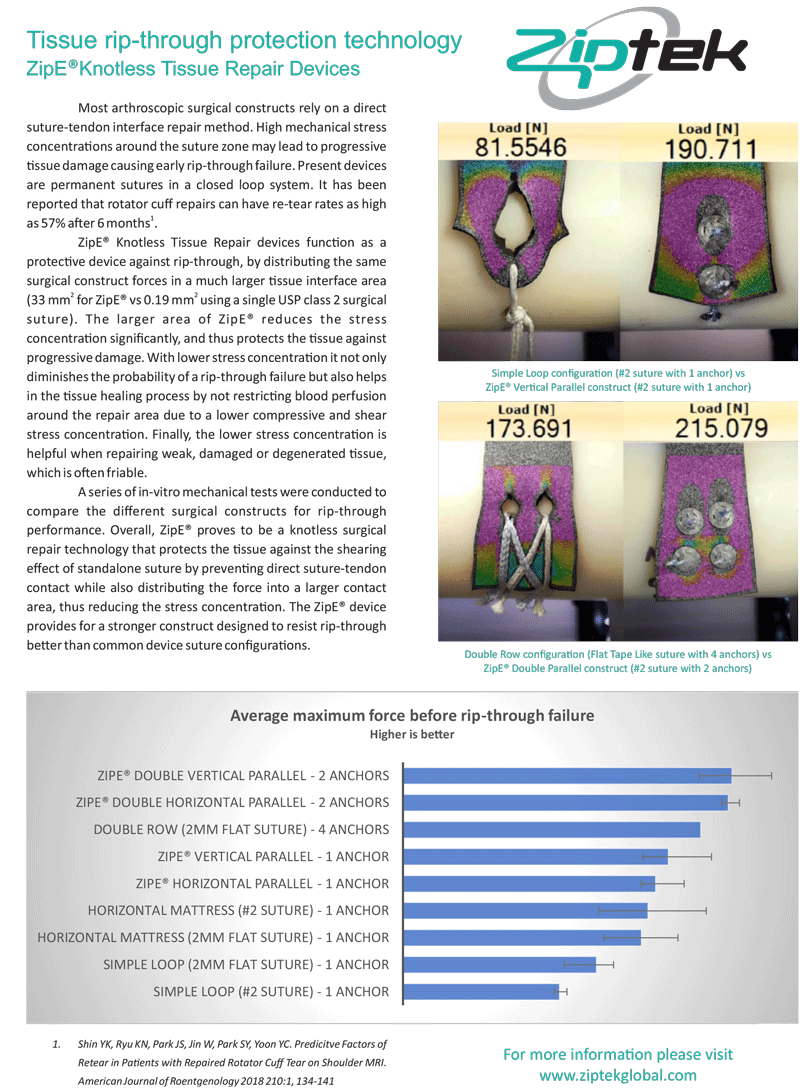
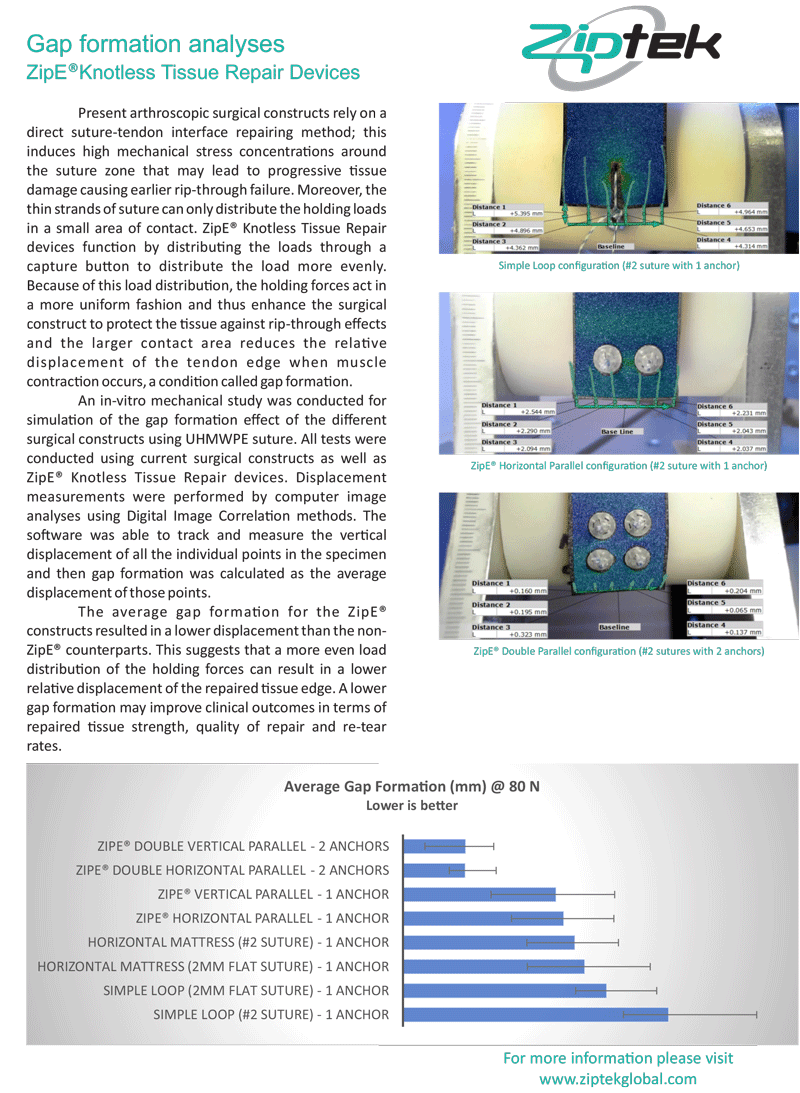
 protects rotator cuff repairs from tendon impingement with its low profile resorbable button
protects rotator cuff repairs from tendon impingement with its low profile resorbable button
 vs simple loop tape-like-suture, weak rubber
vs simple loop tape-like-suture, weak rubber
 vs vs horizontal mattress tape-like/suture, weak rubber
vs vs horizontal mattress tape-like/suture, weak rubber
 vs double row strong rubber
vs double row strong rubber

4.5 mm titanium screw in pig bone exceeds 600 newtons pullout strength- test stopped for safety reasons.

Testing Equipment

Degradation studies

Degradation bath

Testing facilities

Instron- one of 90 machines worldwide

Various PCF sawbone for degradation studies
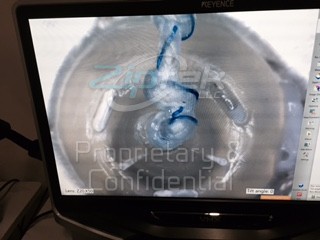
Microscope evaluation of the  device following double row tension and degradation study
device following double row tension and degradation study

Pull out studied

Instron

 biocomposite 6.5 plug intact at 330 newtons in 30 PCF sawbone-suture failure
biocomposite 6.5 plug intact at 330 newtons in 30 PCF sawbone-suture failure
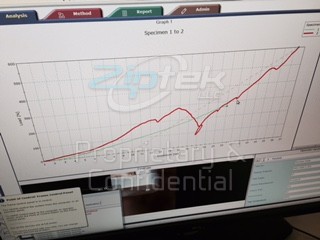
4.5mm titanium  screw in pig bone, exceeds 600 newtons
screw in pig bone, exceeds 600 newtons
